 #News
#News
When should medications be deprescribed?
Physicians should suspend prescription of cardiovascular medications when there could be potential harm or if there are better alternatives, warn researchers
 Researchers from Austria and the US advocate for more in-depth studies into when and how doctors should stop prescribing medications for cardiovascular conditions | Image: Shutterstock
Researchers from Austria and the US advocate for more in-depth studies into when and how doctors should stop prescribing medications for cardiovascular conditions | Image: Shutterstock
A cornerstone of clinical practice, prescription of medications is based on evidence obtained from clinical studies. These, however, usually test the adoption of new, continual-use medications in short-term trials.
Much less attention is paid to the question of discontinuing pharmacological treatments, particularly after a period of treatment in which the patient ages and illnesses can progress, and new conditions can emerge.
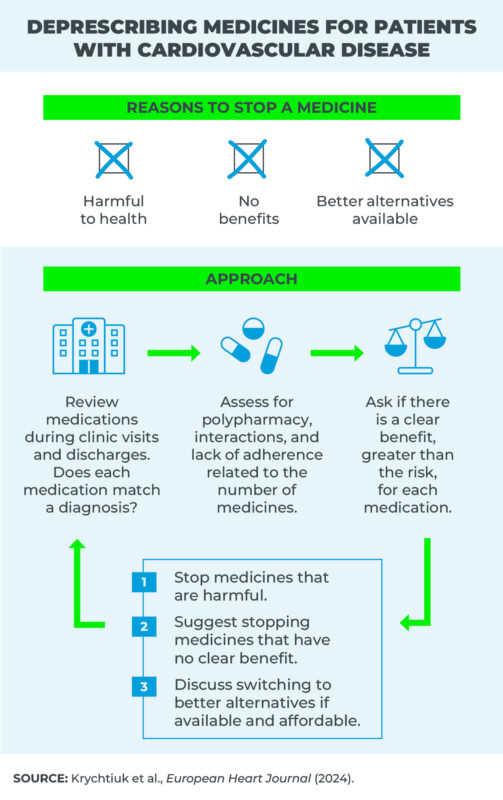
Given the scarcity of data, the clinical practice guidelines offer little or no guidance on when and how to deprescribe medications for cardiovascular conditions.
These decisions are often left to the discretion of clinicians, who, together with their patients, decide to stop certain treatments due to side effects.
“Even in the absence of adverse effects, continuation of medications with no proven effect may cause harm due to drug-drug interactions, the advent of polypharmacy, and additional spending that already overwhelmed health systems could avoid,” argue researchers from the US and Austria in an article in the European Heart Journal, of the European Society of Cardiology.
Types of medications
The publication lists medication types and the respective conditions for which they should be discontinued due to the harm caused to patients, for having no proven effect, or when better options are available.
The authors also outline an approach to be applied by clinics to discontinue certain treatments, taking patient expectations into account.
Patients with advanced heart failure, for example, often express a preference for quality rather than extension of life, which exposes the potential discrepancy between doctor and patient expectations, say the authors.
“Moreover, each patient may feel the severity of side effects in different ways, such as fatigue and erectile dysfunction.”
By way of example among the 11 treatments that may cause harm, the researchers list antiplatelets in patients with stabilized cardiovascular disease taking anticoagulants orally. Evidence demonstrates that this approach increases the risk of hemorrhage, without reducing that of ischemic events.
A fish oil supplement to reduce the risk of heart disease is one of the seven treatments recommended for discontinuation by the authors due to a lack of any proven benefit.
In turn, drugs with better alternatives include warfarin in cases of atrial fibrillation, and clopidogrel after acute coronary syndrome. According to the authors, these and a further four treatments can be replaced by others with more benefits.
According to the authors, a significant obstacle to the deprescription of medications is the absence of quality evidence on the theme.
This includes the lack of information on the process and timeline for discontinuation, the correct strategy (whether to replace, or cease partially or totally), the absence of data on the efficacy of medications in elderly people with comorbidities, and of information about drug interaction.
The authors advocate that committees formulating clinical practice guides should include a regular, structured review of medicines to be used, including deprescription as a guideline to be followed.
Furthermore, research funding should be provided to look into scalable strategies for the procedure.
In the article, the researchers state that clinics have a duty to ensure that their patients are treated with up-to-date, evidence-based medicines in order to reduce morbidity and mortality, and improve quality of life.
They go on to say that it is as or more important to prevent unnecessary harm caused by drugs or interventions proven to be capable of having adverse effects on patients.
*
This article may be republished online under the CC-BY-NC-ND Creative Commons license.
The text must not be edited and the author(s) and source (Science Arena) must be credited.


