 #Essays
#Essays
The frontiers of cancer treatment
New inhibitors revolutionize cancer treatment, but tumor cell resistance demands a different approach
 Heterogeneity in tumor microenvironment challenges therapeutic resistance of cancer cells | National Cancer Institute - Unsplash
Heterogeneity in tumor microenvironment challenges therapeutic resistance of cancer cells | National Cancer Institute - Unsplash
Cancer is often associated with dysregulation of cell signaling pathways, leading to a proliferation of tumor cells.
Over the past twenty years, the development of personalized and targeted pharmacological inhibitors of these pathways has revolutionized oncological treatment.
A notable example is the MAPK (mitogen-activated protein kinase) cell signaling pathway, which regulates various processes and is frequently altered in tumors such as melanoma and colorectal cancer, for which drugs such as vemurafenib and cetuximab have proven effective, improving patient survival and managing cancer progression.
However, the outcomes of these treatments are often limited by the development of resistance by the tumor.
This resistance arises from the dynamic evolution of heterogeneous cancer cell populations, which interact with each other and with the tumor microenvironment.
Genetic heterogeneity promotes the selection of resistant cells, allowing for the emergence of secondary mutations that reactivate oncogenic pathways and compromise therapeutic success.
A counterintuitive response to this challenge has emerged: is it possible to induce cytotoxicity by increasing oncogenic signaling beyond the limit tolerated by cancer cells? Preclinical studies suggest it is.
The science of hyperactivation
Hyperactivation of oncogenic pathways can generate such intense cellular stress that the tumor cells are unable to survive.
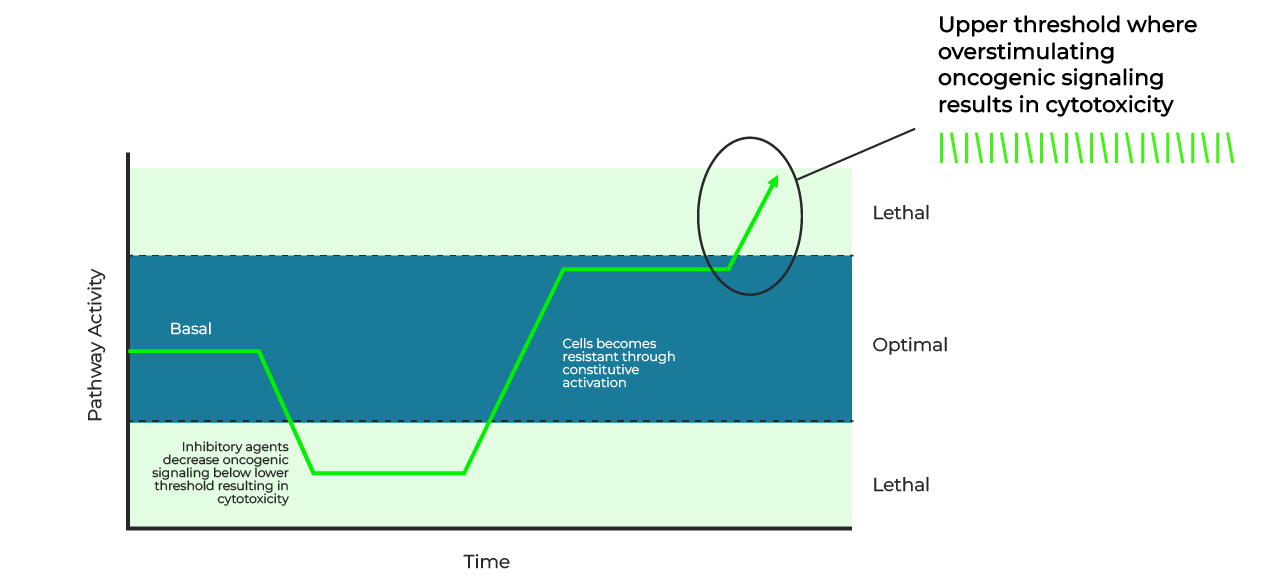
The approach is based on the fact that cancer cells maintain pathway signaling at an “optimal” level, enough to sustain growth without causing cell collapse.
Disrupting this balance, whether via inhibition or hyperactivation, exposes vulnerabilities that can be exploited therapeutically.
Recent research has investigated treatment combinations that alternate between inhibition and hyperactivation with the aim of impacting heterogeneous tumor cell populations.
These strategies have the potential to reduce tumor burden and restrict cancer growth, even in cases resistant to target-directed treatments.
When cells develop resistance to inhibitors, they often compensate with constant activation of the pathway, moving toward an upper signaling threshold.
Paradoxically, this adaptation creates a vulnerability: hyperactivation beyond this threshold triggers fatal cellular stress.
Therapies that exploit an alternating pattern between inhibition and hyperactivation can thus destabilize cancer cells, inducing cell death or contributing to the generation of less aggressive resistant clones.
Although the approach is promising, there are significant challenges yet to overcome. One of the biggest obstacles is the lack of drugs developed specifically to hyperactivate oncogenic pathways.
In recent decades, the primary focus has been on developing inhibitors, leaving a gap in the research on compounds that stimulate hyperactivation.
This limitation, however can be seen as an opportunity.
Drugs already approved for other objectives, such as tissue regeneration or protection, can be repositioned for this purpose.
Advances in the identification and development of new compounds also offer potential for the creation of a new class of drugs.
Innovative approach
Determining the exact thresholds for switching between inhibition and hyperactivation remains a growing field of study.
Identifying these thresholds is crucial to developing safe and effective clinical protocols.
Furthermore, the combination of approaches, including drug repositioning and development of new agents, could accelerate the progression of this strategy to clinical trials.
Exploring the upper and lower thresholds of cellular signaling not only challenges existing paradigms, it also offers new perspectives on treating resistant cancers, providing alternatives for patients with few therapeutic options.
This innovative approach thus has the potential to improve therapeutic interventions for cancer.
Julia Rezende da Silva is a PhD student on the Postgraduate Program in Pharmacy (Pathophysiology and Toxicology) at the School of Pharmaceutical Sciences of the University of São Paulo, where she is studying genes involved in cancer progression and resistance. She did a supervised fellowship at the Institute of Cancer Research in London, United Kingdom, where she studied the hyperactivation of oncogenic pathways as a new therapeutic strategy for treating cancer.
Opinion articles do not necessarily reflect the views of Science Arena or Hospital Israelita Albert Einstein.
*
This article may be republished online under the CC-BY-NC-ND Creative Commons license.
The text must not be edited and the author(s) and source (Science Arena) must be credited.


