 #News
#News
To reduce costs and increase diversity in clinical studies
A US regulatory agency advocates analyzing treatment efficacy by combining observational studies with targeted data collection
 As stated in an article published in the scientific journal JAMA, pragmatic clinical trials feature "simplified, prospective designs, tailored to address a focused research question. While these trials may occasionally employ randomization, alternative design methodologies can also be utilized." | Image: Shutterstock
As stated in an article published in the scientific journal JAMA, pragmatic clinical trials feature "simplified, prospective designs, tailored to address a focused research question. While these trials may occasionally employ randomization, alternative design methodologies can also be utilized." | Image: Shutterstock
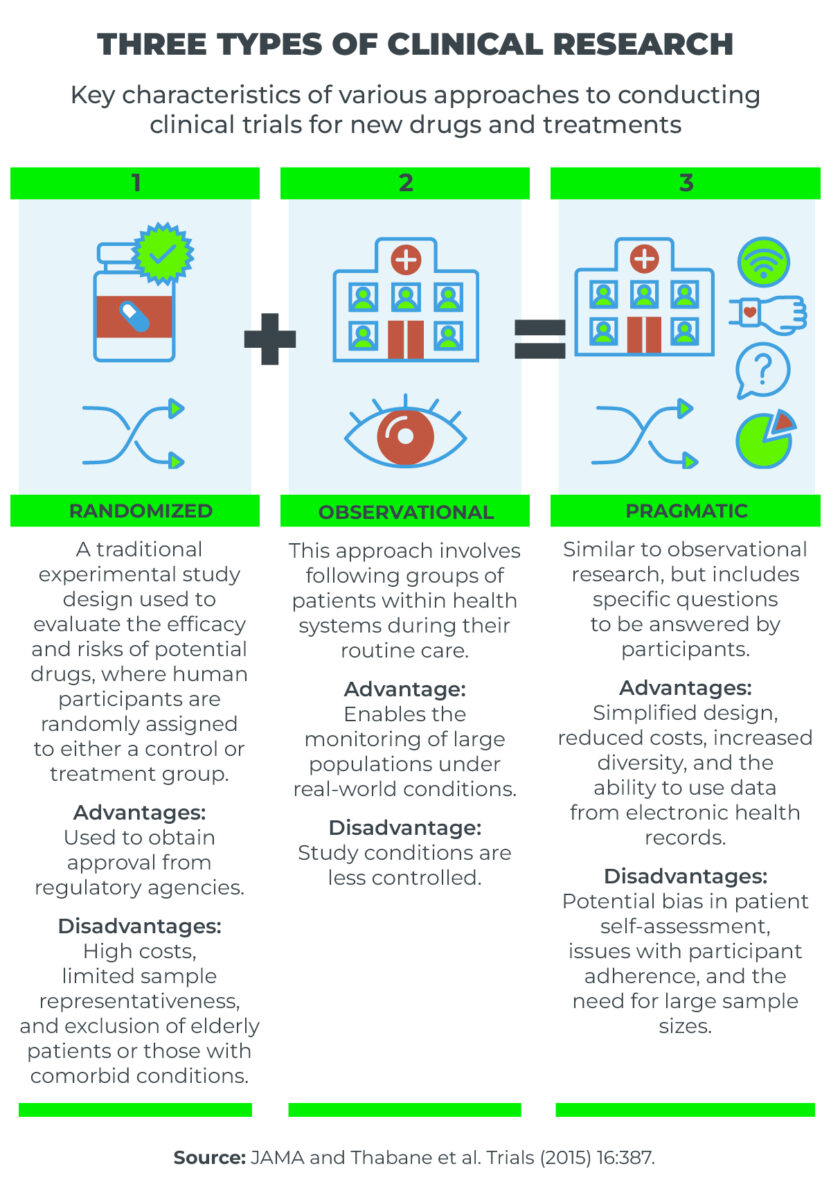
Randomized clinical trials are essential for the approval of new drugs and treatments by regulatory agencies, such as Brazil’s National Health Surveillance Agency (ANVISA) and the United States Food and Drug Administration (FDA).
These trials are often complex and costly, which can make their implementation unfeasible in certain cases.
In contrast, observational studies monitor patients undergoing specific treatments to assess health conditions and the effects of the drugs used.
While observational studies offer less control over variables, they provide the advantage of real-world data, encompassing a larger patient population. This can be particularly useful for identifying adverse effects that, while significant, may be rare.
In an article published in the JAMA journal, by the American Medical Association, professionals from the FDA advocate for a middle ground between these two trial types: namely, pragmatic clinical research.
According to the authors, this research approach warrants greater attention and focus.
“[This type of clinical research] features simplified, prospective designs, tailored to address a focused research question. While these trials may occasionally employ randomization, alternative design methodologies can also be utilized,” wrote Ali Abbasi, Lesley Curtis, and Robert Califf.
The authors suggest that an evidence generation system integrated into routine treatment practices following the initial approval of drugs could be highly promising for assessing risks and benefits—an aspect that is not always fully addressed in the current evaluation framework.
High costs and limited diversity
The authors of the article highlight that this type of research holds significant promise when integrated into health systems, offering researchers a means to circumvent challenges commonly associated with traditional clinical trials.
Among these challenges are the often prohibitively high costs and the limited diversity that can arise when trials are conducted in centers that do not represent the full spectrum of care and patient populations.
The paper suggests that an evidence generation system integrated into routine treatment practices following the initial approval of drugs could be highly promising for assessing risks and benefits—an aspect not always fully addressed in the current evaluation framework.
This is particularly relevant in numerous therapeutic areas, especially for common chronic illnesses such as cardiovascular, pulmonary, and renal conditions, as well as type 2 diabetes and Alzheimer’s disease.
In these cases, the authors note, large-scale clinical studies are essential, as treatment effects are often modest, and the risks and benefits are distributed heterogeneously across the affected populations.
According to the article, the FDA is particularly interested in pragmatic clinical research following the initial approval of treatments in several key areas.
“There is a need for robust data to inform clinical practice, particularly post-market confirmatory studies,” the authors emphasize.
“These trials may more closely reflect real-world practice conditions than traditional clinical trials. They can also utilize more pragmatic designs to focus on the critical elements needed to confirm benefits and risks for the intended populations,” they write.
To recruit a large and diverse patient sample at a lower cost, alongside the simplified study design, the authors propose leveraging electronic health records, wearable sensors—which collect health data from users—and virtual methods integrated into clinical practice as data sources.
*
This article may be republished online under the CC-BY-NC-ND Creative Commons license.
The text must not be edited and the author(s) and source (Science Arena) must be credited.


